The effect of fillers on the overall migration resistance and mechanical properties of food-grade natural rubber gloves
Vol. 19., No.9., Pages 929-945, 2025
DOI: 10.3144/expresspolymlett.2025.70
DOI: 10.3144/expresspolymlett.2025.70
GRAPHICAL ABSTRACT
ABSTRACT
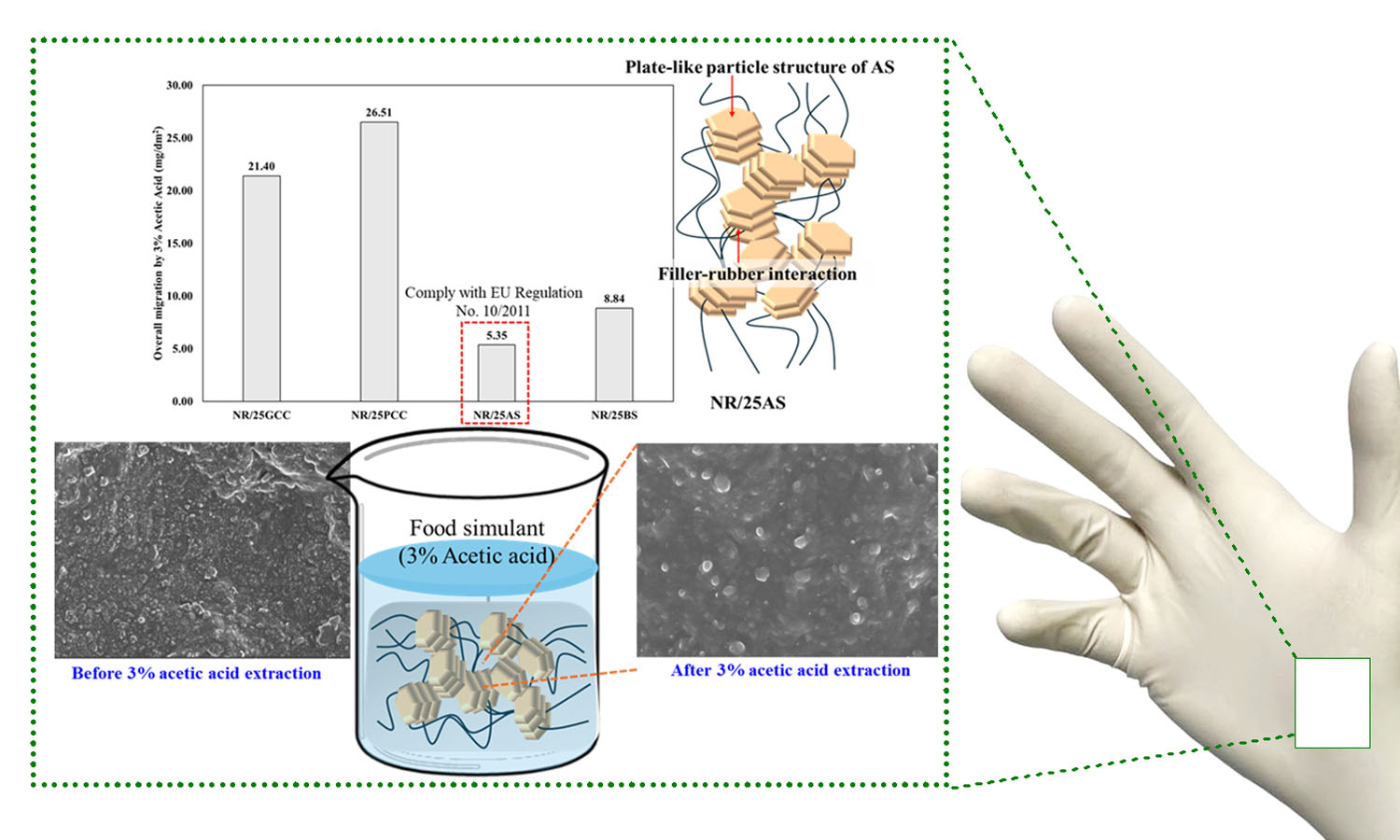
Powder-free natural rubber gloves for chemical migration resistance of food-contact grade are prepared using a variety of fillers, including ground calcium carbonate (GCC), precipitated calcium carbonate (PCC), aluminum silicate (AS), and barium sulfate (BS)-filled natural rubber (NR), respectively. The properties of NR gloves, including mechanical, dynamic mechanical, and thermal properties, were investigated. Furthermore, the overall migration test of NR gloves was conducted according to the regulations for food contact gloves (EU Regulation No. 10/2011), using 3% acetic acid as the simulant. Among the fillers studied, the plate-like particles of AS facilitated the most effective filler-rubber interactions and reinforcement in AS-filled natural rubber (NR/AS). Consequently, the highest crosslink density, force at break, and damping properties of NR gloves were achieved by applying AS in the NR matrix. Moreover, the lowest overall migration level was observed for NR/AS with a value of 5.35 mg/dm2, which complies with EU Regulation (overall migration of food simulants shall not exceed 10 mg/dm2). Therefore, NR gloves filled with AS are suitable for food-contacting NR gloves.
RELATED ARTICLES
Reinforcing effect of thermo-oxidative reclaimed rubber on NR/SBR blends for tire tread applications
Yunhui Xu, Zaheer ul Haq, Junrong Li, Hui Tu, Zaixue Wang, Houluo Cong
Vol. 20., No.2., Pages 142-153, 2026
DOI: 10.3144/expresspolymlett.2026.12
Vol. 20., No.2., Pages 142-153, 2026
DOI: 10.3144/expresspolymlett.2026.12

This study explores the application of thermo-oxidative reclaimed ground tire rubber (RGTR) in natural rubber (NR)/styrene butadiene rubber (SBR) composite, focusing on its impact on morphology, mechanical properties, rheological behavior, vulcanization characteristics, aging resistance, tear strength and abrasion resistance. The findings revealed that RGTR enhances the tear strength and abrasion resistance of NR/SBR composites while maintaining comparable tensile strength, elongation at break, and modulus. The incorporation of RGTR reduced Mooney viscosity of the NR/SBR composites and improved flowability. It also shortened the vulcanization time and enhanced vulcanization efficiency. The NR/SBR composites with RGTR loadings below 60 phr exhibited optimal performance, achieved a maximum tear strength of 93.77 N/mm and improved abrasion resistance. However, higher RGTR content led to increased agglomeration, as evidenced by scanning electron microscopy (SEM), which showed finer dispersion at lower RGTR contents and larger aggregates at higher loadings. These findings demonstrate the potential of RGTR as a sustainable additive for enhancing specific properties in NR/SBR composites, contributing to both performance optimization and waste tire management.
Dibyendu Dey, Sharmistha Dhar, Barkat Aziz, Sambhu Bhadra, Sujith Nair, Kinsuk Naskar
Vol. 20., No.2., Pages 127-141, 2026
DOI: 10.3144/expresspolymlett.2026.11
Vol. 20., No.2., Pages 127-141, 2026
DOI: 10.3144/expresspolymlett.2026.11

Ground sugarcane bagasse (GSB), an agro-waste rich in lignocellulosic components, was studied as a sustainable bio-filler in natural rubber (NR) tread compounds to lessen reliance on petroleum-derived carbon black (CB). A control formulation with 45 phr CB was compared to hybrid formulations with 40, 35, and 30 phr CB mixed with 5, 10, and 15 phr GSB. Tensile strength 13.1 MPa, elongation at break 700%, and hardness 67 Shore A were all optimally balanced by the compound containing 10 phr GSB (S2), while also exhibiting good cure behavior and thermal stability. Improved tire performance characteristics were confirmed by a dynamic mechanical study, which showed that tan δ at 60 °C decreased by 8.0% (resulting in lower rolling resistance) and increased by 3.9% (improving wet traction) at 0°C. The Payne effect showed improved filler dispersion as a result of GSB partially replacing CB. The results show that appropriately dispersed GSB can partially reinforce NR, enhancing energy efficiency and sustainability. However, larger GSB loadings decrease modulus, tear strength, and abrasion resistance due to lower interfacial adhesion and the presence of micro-voids. According to this study, pulverized sugarcane bagasse shows promise as an environmentally friendly filler for green tire applications, promoting the circular economy and lowering the carbon footprint of rubber compounding.
Xavier Colom, Scott Martínez, Fernando Carrillo-Naverrete, Javier Cañavate
Vol. 20., No.2., Pages 114-126, 2026
DOI: 10.3144/expresspolymlett.2026.10
Vol. 20., No.2., Pages 114-126, 2026
DOI: 10.3144/expresspolymlett.2026.10

The need to recycle elastomeric waste requires studying its viability in industrial applications. This study investigates the feasibility of recycling elastomeric waste by analyzing whether virgin ethylene-propylene-diene monomer (EPDM) can be replaced by blends of virgin EPDM and thermomechanically and microwave devulcanized EPDM (EPDMd) in industrial applications from the perspective of environmental degradation. Two types of samples were examined: conventional EPDM used to roof membranes, and EPDM blended with different amounts (20, 40, and 50 phr) of EPDMd. Samples were subjected to natural aging in coastal and mountainous environments. Results show that mechanical properties decline with higher EPDMd content and, to a lesser degree, with prolonged outdoor exposure. The coastal climate proved more aggressive than the mountainous one when EPDMd content exceeded 40 phr. Zinc stearate (ZnSt2), a byproduct of vulcanization, was found to influence the evolution of the mechanical behavior. The combined analysis of scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), abrasion tests, and thermogravimetric analysis (TGA) provided insights into the degradation processes of these elastomeric blends.
Rattanawadee Ninjan, Bencha Thongnuanchan, Phakawat Tongnuanchan, Subhan Salaeh, Jutharat Intapun, Abdulhakim Masa, Natinee Lopattananon
Vol. 20., No.1., Pages 18-35, 2026
DOI: 10.3144/expresspolymlett.2026.3
Vol. 20., No.1., Pages 18-35, 2026
DOI: 10.3144/expresspolymlett.2026.3

The present study has proposed a straightforward method to improve the reprocessability of modified natural rubber (NR) by blending it with gelatin (GT). The reprocessable characteristics of these blends were evaluated based on their remolding capabilities and mechanical recovery performance. In this method, poly(vinylbenzyl chloride) (PVBC) was first grafted onto NR chains to create graft copolymers known as NR-g-PVBC. The benzyl chloride groups in the graft copolymers were subsequently converted into quaternary ammonium groups, referred to as NR-g-QPVBC. This modification enabled ionic crosslinking when NR-g-QPVBC reacted with ethylenediamine tetraacetic acid. Blends were created by incorporating GT powder into the NR-g-QPVBC latex. The optimal loading level of GT was determined to be 30 wt%, as the resulting film exhibited the highest recovery of tensile properties. Initially, the film's tensile strength was measured at 15 MPa. After being remolded at 160 °C, the tensile strength decreased to 9.3 MPa, resulting in a recovery rate of 60.7% and withstanding a tensile strain of 144%. Although the NR-g-QPVBC/GT films could be remolded, their tensile properties declined with increasing remolding cycles. Therefore, this work demonstrated a practical method for producing NR-based films that could be reshaped through hot-pressing after being formed into products, increasing their reusability.
Hatay Cöcen, Nilgün Kızılcan
Vol. 20., No.1., Pages 82-96, 2026
DOI: 10.3144/expresspolymlett.2026.7
Vol. 20., No.1., Pages 82-96, 2026
DOI: 10.3144/expresspolymlett.2026.7

This study investigates a sustainable hybrid-filler strategy for natural rubber (NR) compound by partially replacing petroleum-based carbon black (CB) with talc and introducing a silane coupling agent to mitigate interfacial incompatibility. Compounds containing CB, CB+talc and CB+talc+increasing silane were produced via two-stage mixing and characterized for morphology (dispersion/mapping), curing and flow behavior (differential scanning calorimetry DSC/moving die rheometer, MDR/Mooney), crosslink density (Flory–Rehner), physical–mechanical properties, dynamic performance (Payne effect/heat build-up/tension–fatigue), and thermal stability (aging/thermogravimetric analysis,TGA). Talc reduced the compound viscosity, offering processing benefits. The swelling test indicated that talc decreased crosslink density, but silane recovered it, forming covalent linkages. Tensile strength and elongation at break were improved without altering hardness. Dynamically, talc increased heat build-up, whereas silane inverted the trend and reduced the temperature rise gradually from 41.5 to 29.4°C at 2 phr. Fatigue life was improved with talc (~10%), and further with silane (up to 36% at 2 phr), highlighting a favorable stiffness–fatigue balance with compatibilization. Overall, partial CB replacement by talc, in combination with silane, delivers meaningful sustainability gains with improved dynamic performance while preserving key mechanical properties of NR compounds.




