Fe₃O₄@ZIF-8 core–shell nanocatalysts for highly efficient and magnetically recyclable glycolysis of PET waste
Longqiang Xiao, Weijia Huang, Kaihong Lin, Shucui Han, Zuyun Luo, Linxi Hou, Yan’gen LV
Vol. 20., No.1., Pages 3-17, 2026
DOI: 10.3144/expresspolymlett.2026.2
DOI: 10.3144/expresspolymlett.2026.2
GRAPHICAL ABSTRACT
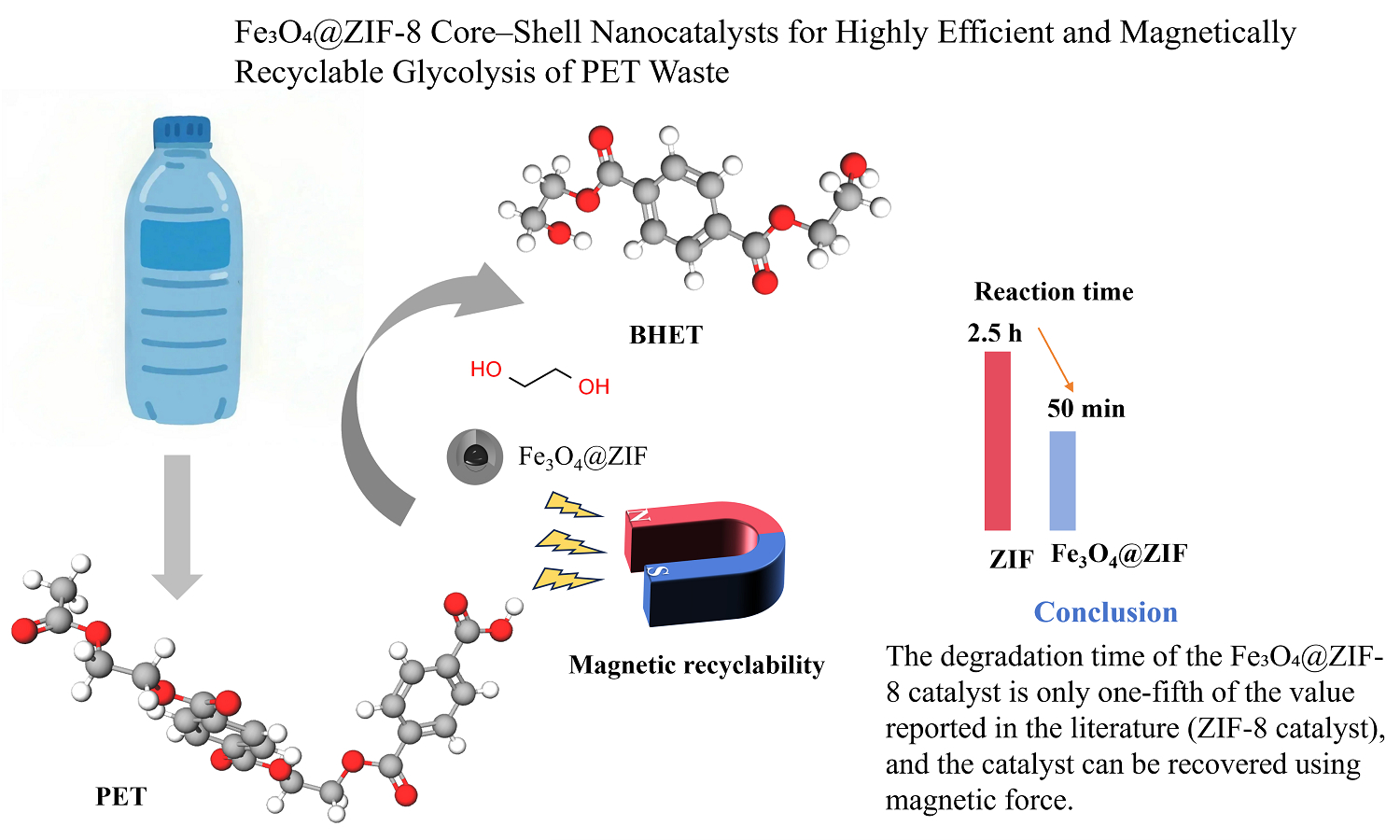
ABSTRACT
In this study, magnetic core-shell Fe3O4@ZIF-8 was synthesized via a hydrothermal method and applied to Polyethylene terephthalate(PET) degradation. The catalytic degradation of PET by Fe3O4@ZIF-8 was carried out under atmospheric pressure, yielding high-value bis(2-hydroxyethyl) terephthalate (BHET) monomers. The as-synthesized Fe3O4@ZIF-8 core-shell composites possess hierarchical porosity with tunable nanoscale cavities. SEM and TEM analyses confirmed the core-shell morphology, with nanoparticles having a size distribution of 180–280 nm. The degradation product was identified as a high-purity, colorless, and transparent monomeric BHET through 1H NMR and LC analyses. Based on a series of onefactor experiments and a Box-Behnken experimental design, the optimal process conditions were determined to be an alcoholysis temperature of 200°C, a catalyst dosage of 0.5 wt% (relative to PET mass), a reaction time of 50 min, and an ethylene glycol-to-PET mass ratio of 4.5:1. Under these conditions, the actual BHET yield reached 81.12%, closely matching the predicted value.
RELATED ARTICLES
Xavier Colom, Scott Martínez, Fernando Carrillo-Naverrete, Javier Cañavate
Vol. 20., No.2., Pages 114-126, 2026
DOI: 10.3144/expresspolymlett.2026.10
Vol. 20., No.2., Pages 114-126, 2026
DOI: 10.3144/expresspolymlett.2026.10
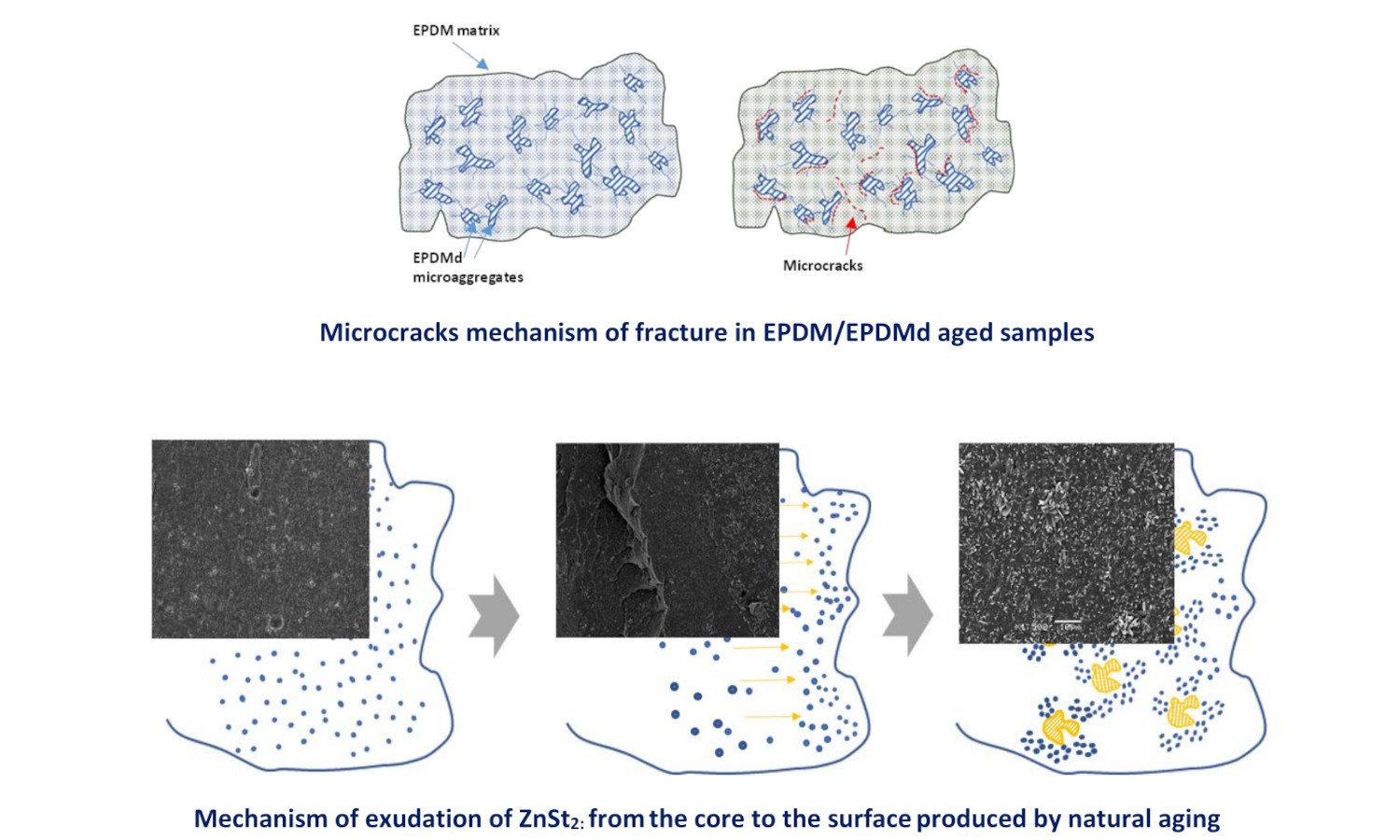
The need to recycle elastomeric waste requires studying its viability in industrial applications. This study investigates the feasibility of recycling elastomeric waste by analyzing whether virgin ethylene-propylene-diene monomer (EPDM) can be replaced by blends of virgin EPDM and thermomechanically and microwave devulcanized EPDM (EPDMd) in industrial applications from the perspective of environmental degradation. Two types of samples were examined: conventional EPDM used to roof membranes, and EPDM blended with different amounts (20, 40, and 50 phr) of EPDMd. Samples were subjected to natural aging in coastal and mountainous environments. Results show that mechanical properties decline with higher EPDMd content and, to a lesser degree, with prolonged outdoor exposure. The coastal climate proved more aggressive than the mountainous one when EPDMd content exceeded 40 phr. Zinc stearate (ZnSt2), a byproduct of vulcanization, was found to influence the evolution of the mechanical behavior. The combined analysis of scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), abrasion tests, and thermogravimetric analysis (TGA) provided insights into the degradation processes of these elastomeric blends.
Zaheer ul Haq, Teng Ren, Xinyan Yue, Krzysztof Formela, Denis Rodrigue, Xavier Colom Fajula, Tony McNally, Dong Dawei, Yong Zhang, Shifeng Wang
Vol. 19., No.3., Pages 258-293, 2025
DOI: 10.3144/expresspolymlett.2025.20
Vol. 19., No.3., Pages 258-293, 2025
DOI: 10.3144/expresspolymlett.2025.20
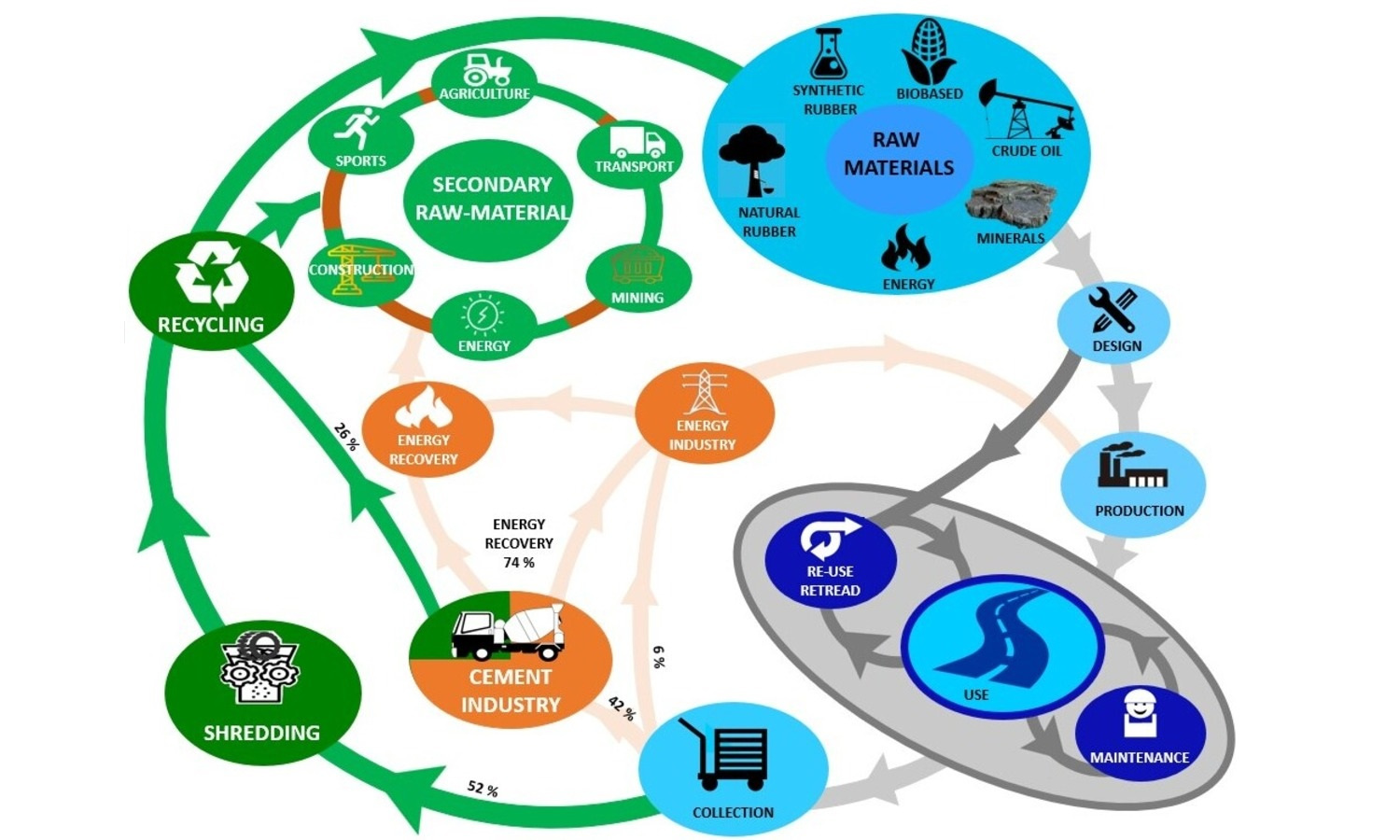
As a complex composite material, tire rubber has always presented significant environmental and waste management concerns due to its non-biodegradability and accumulation in landfills. The devulcanization of tire rubber has emerged as a historical challenge in the field of sustainable rubber engineering since Goodyear invented cross-linking in 1839. This review provides a comprehensive analysis of waste tire recycling processes, focusing on the sources, legislation, management strategies, and utilization across different regions. It explores the multifaceted challenges of devulcanizing rubber, with a specific focus on transitioning from ground tire rubber to the concept of multi-decrosslinking: sulfur bridge breakage, rubber chain depolymerization and micro-nano sized core-shell carbon black. Ideal devulcanization has restricted the release of reinforcing fillers, resulting in devulcanized rubber mainly containing dozens of micron particles, which hinder the wide usage of devulcanized rubber. This review comprehensively assesses the current state-of-the-art techniques for tire rubber devulcanization, including physical, chemical and biological methods. It explores the intricacies of ground tire rubber as a starting material, structural evolution of ground tire rubber during the devulcanization process and the associated challenges in achieving efficient devulcanization while retaining desirable mechanical properties. Furthermore, through an in-depth analysis of recent advancements, limitations and prospects, this paper offers a complete understanding of the challenges faced in tire rubber devulcanization. Considering the technical and environmental aspects of these processes, this work contributes to multi-decrosslinking, the ongoing discourse on sustainable materials development and circular economy initiatives, which pave the way for future innovations in the field of rubber recycling.
Ahmed Nasr, Petr Svoboda
Vol. 18., No.3., Pages 309-325, 2024
DOI: 10.3144/expresspolymlett.2024.22
Vol. 18., No.3., Pages 309-325, 2024
DOI: 10.3144/expresspolymlett.2024.22
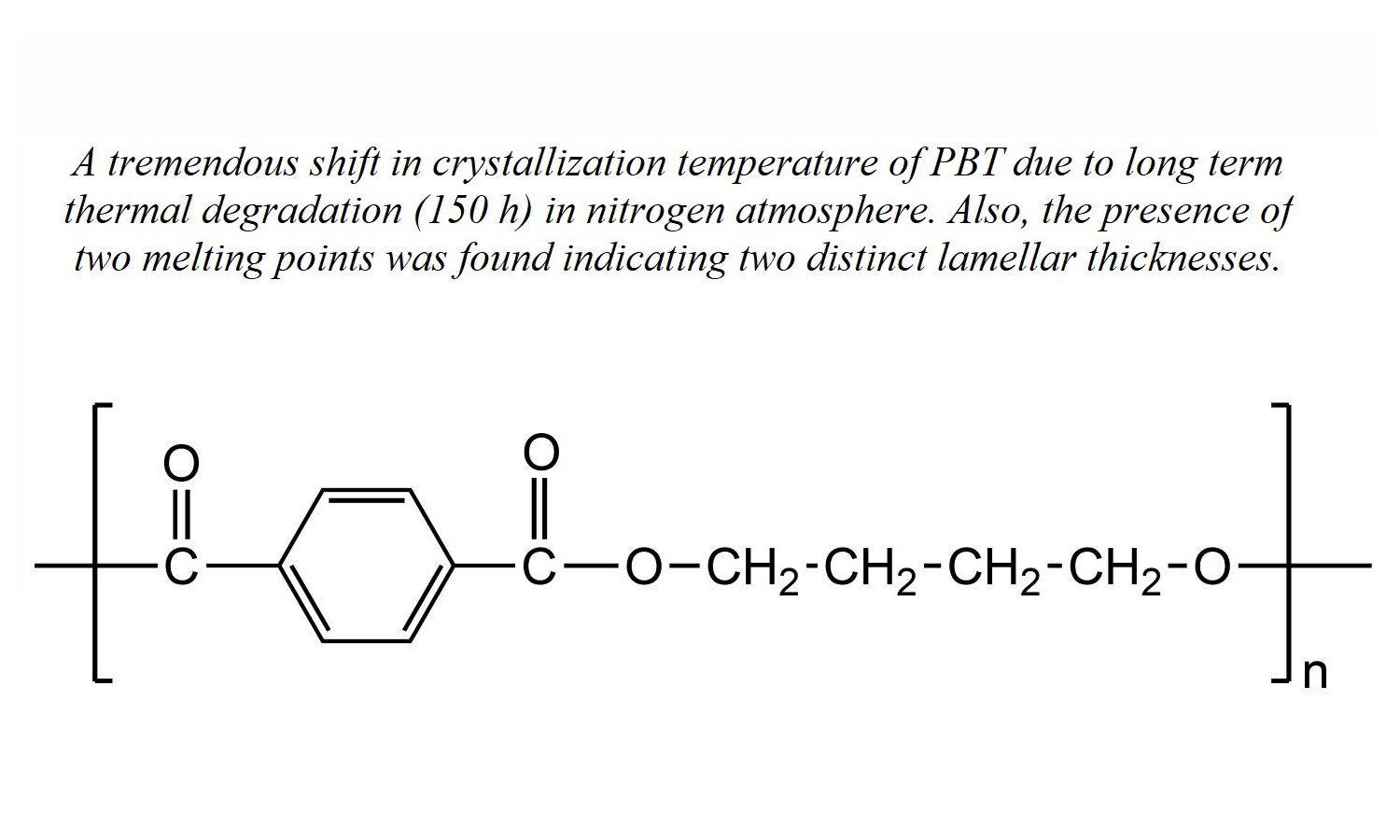
Our work reveals a notable shift in the crystallization temperature (Tc) of poly(butylene terephthalate) (PBT) at which crystallization occurs due to exposure to prolonged thermal degradation at 270°C in an environment of nitrogen gas. The initial Tc of 193°C undergoes a marked decrease, settling at 133°C, which signifies a considerable 60°C shift towards lower temperature ranges. This transition is discernible across three distinct degradation stages: an initial phase of increase, an intermediate phase characterized by a sharp decline, and a subsequent late stage of the degradation phase characterized by a more moderate decrease in Tc. Both crystallinity and crystallization kinetics consistently mirror this pattern, demonstrating an initial rise, a rapid subsequent drop, and a gradual decline in the late-stage period. Evident from the presence of two melting peaks, the research implies differing lamellar thicknesses. As the degradation progresses, the melting points of these peaks, denoted as Tm1 and Tm2, decline at 38 and 41°C, respectively. Validation of the degradation-induced changes is provided by a small angle X-ray scattering (SAXS), which corroborates the observed decrease in the long period (L). A contextualization of the results against prior studies underscores analogous trends in the alteration of crystallization behaviour consequent to degradation.



