Recyclable high-strength polybutadiene-based rubber with self-healing and shape memory properties via dynamic boronic ester and Diels-Alder chemistry
Shengao Yang, Yan Wang, Fang Wang, Kaiyi Zhang, Xinxin Lv, Hao Teng, Rui Zheng, Faliang Luo, Qian Xing
Vol. 19., No.1., Pages 94-106, 2025
DOI: 10.3144/expresspolymlett.2025.7
DOI: 10.3144/expresspolymlett.2025.7
GRAPHICAL ABSTRACT
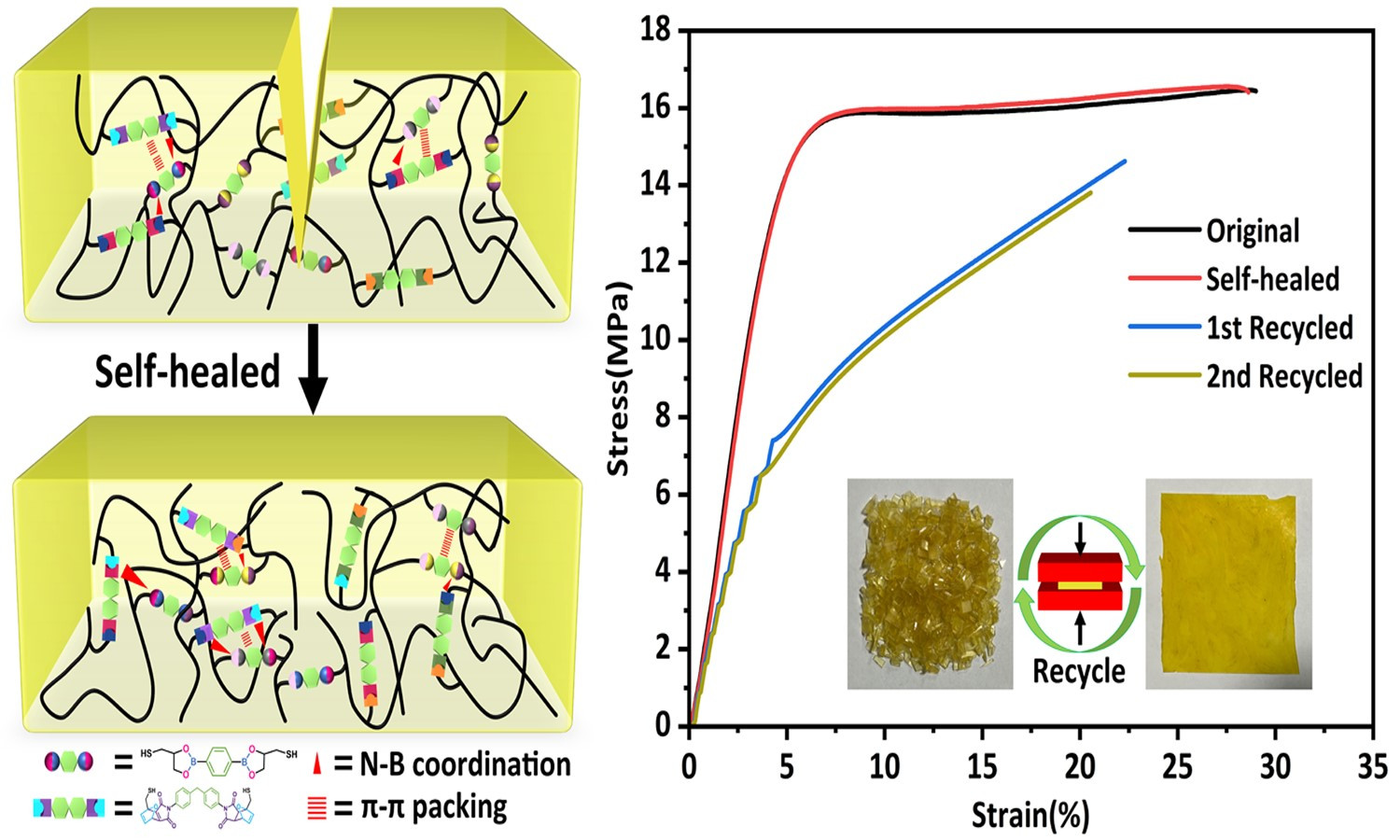
ABSTRACT
Dynamic cross-linked networks (DCNs) endow thermoset rubber with self-healability and recyclability to extend its lifetime and alleviate environmental pollution. However, the contradiction between high self-healing and mechanical properties in DCNs rubber is always difficult to be resolved. Herein, we used boronic ester (BO) and Diels-Alder dynamic covalent bonds (DA) to synthesize polybutadiene-based dual networks rubber (PB-BO-DA) via thiol-ene reaction. This approach achieved a tensile strength of 16.46 MPa and 99% self-healing efficiency, facilitated by extensive intermolecular interactions (π-π packing and N-B coordination) and fully dynamic cross-linking. In addition, multiple dynamic cross-linked networks (MDCNs) polybutadiene-based rubber also show excellent shape memory ability and recyclability. This strategy might open a helpful pathway to fabricate intelligent multifunctional polymers with high strength and high self-healing efficiency.
RELATED ARTICLES
Rattanawadee Ninjan, Bencha Thongnuanchan, Phakawat Tongnuanchan, Subhan Salaeh, Jutharat Intapun, Abdulhakim Masa, Natinee Lopattananon
Vol. 20., No.1., Pages 18-35, 2026
DOI: 10.3144/expresspolymlett.2026.3
Vol. 20., No.1., Pages 18-35, 2026
DOI: 10.3144/expresspolymlett.2026.3
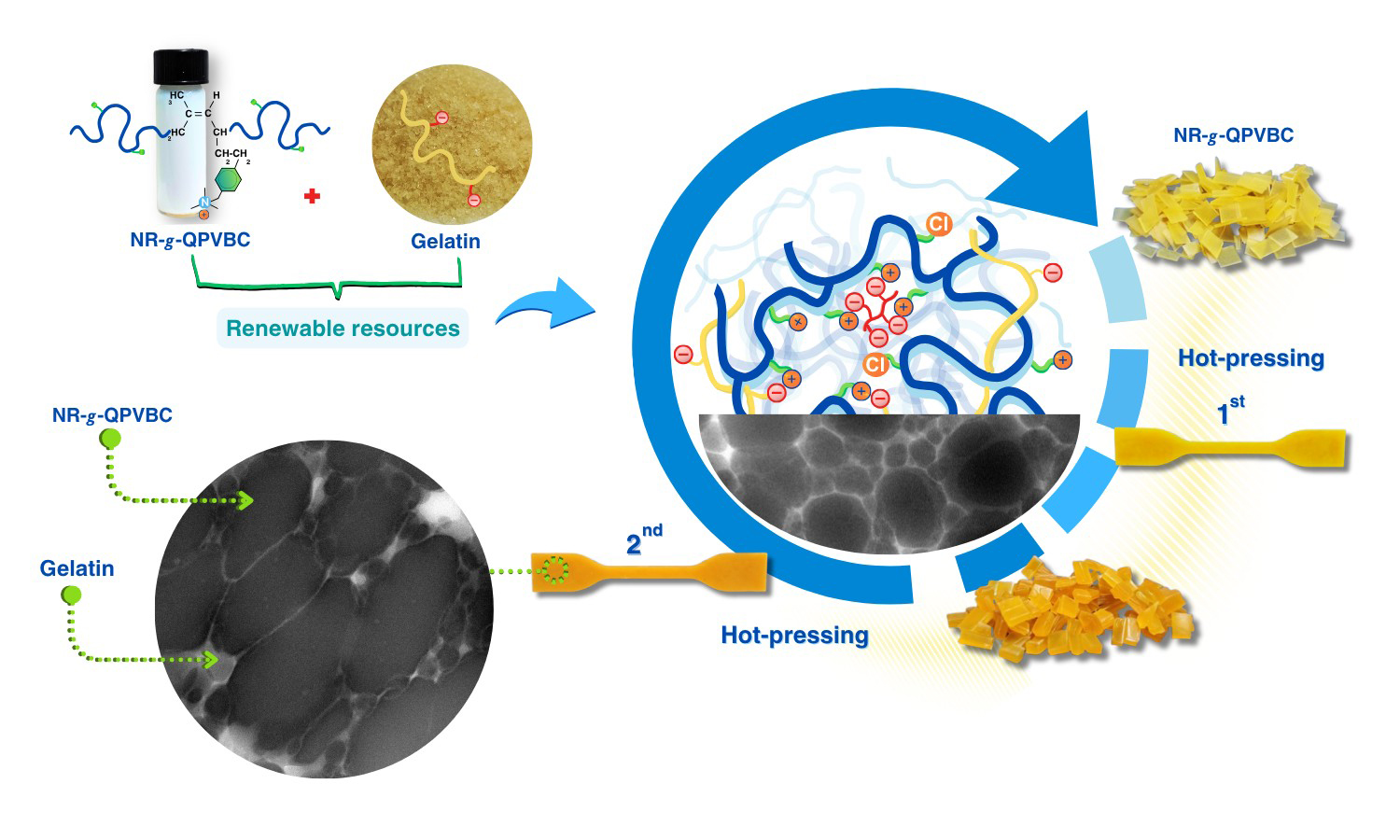
The present study has proposed a straightforward method to improve the reprocessability of modified natural rubber (NR) by blending it with gelatin (GT). The reprocessable characteristics of these blends were evaluated based on their remolding capabilities and mechanical recovery performance. In this method, poly(vinylbenzyl chloride) (PVBC) was first grafted onto NR chains to create graft copolymers known as NR-g-PVBC. The benzyl chloride groups in the graft copolymers were subsequently converted into quaternary ammonium groups, referred to as NR-g-QPVBC. This modification enabled ionic crosslinking when NR-g-QPVBC reacted with ethylenediamine tetraacetic acid. Blends were created by incorporating GT powder into the NR-g-QPVBC latex. The optimal loading level of GT was determined to be 30 wt%, as the resulting film exhibited the highest recovery of tensile properties. Initially, the film's tensile strength was measured at 15 MPa. After being remolded at 160 °C, the tensile strength decreased to 9.3 MPa, resulting in a recovery rate of 60.7% and withstanding a tensile strain of 144%. Although the NR-g-QPVBC/GT films could be remolded, their tensile properties declined with increasing remolding cycles. Therefore, this work demonstrated a practical method for producing NR-based films that could be reshaped through hot-pressing after being formed into products, increasing their reusability.
Cristian Valdés, Valentina Guzmán, Camila Ponce, Maribel Mamani, Juan Guevara, Claudia Vergara, Rodrigo Andler
Vol. 19., No.6., Pages 594-609, 2025
DOI: 10.3144/expresspolymlett.2025.45
Vol. 19., No.6., Pages 594-609, 2025
DOI: 10.3144/expresspolymlett.2025.45
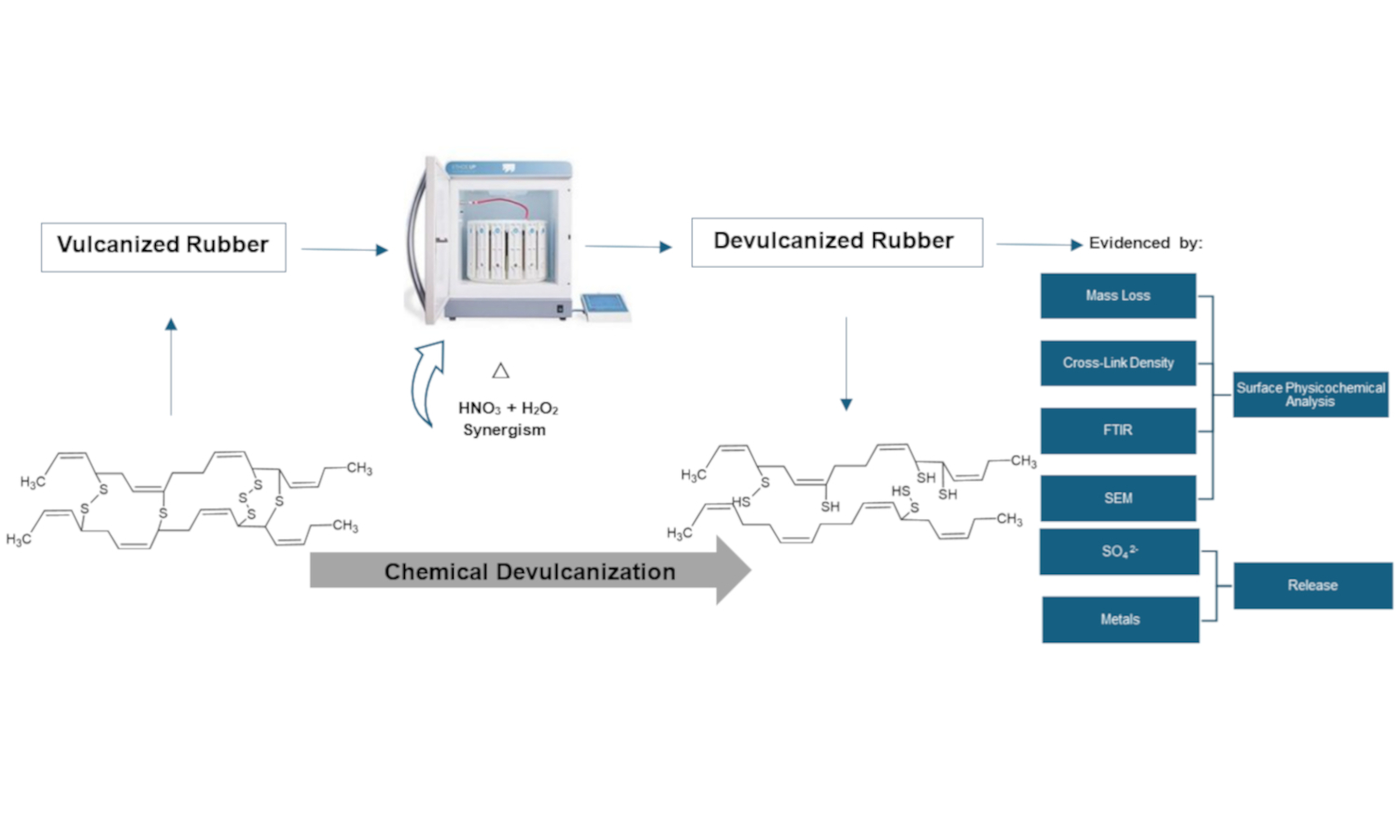
Waste rubber disposal causes considerable negative environmental impacts due to its increase worldwide, mainly in the automotive industry. Therefore, the search for technological solutions for rubber waste is a priority, and the first step in this material degradation is devulcanization due to its difficult degradation. This study evaluated rubber devulcanization using a closed vessel microwave digestion system with nitric acid (HNO3) and hydrogen peroxide (H2O2) through chemical characterization, aiming at verifying the synergistic effect between these oxidizing agents. Microwave irradiation was applied as a heating method to facilitate the chemical reactions, focusing on the synergism between HNO3 and H2O2. Results showed that 5 M H2O2 in combination with 1% HNO3, presented better results. A greater decrease in cross-link density was demonstrated as the concentration of H2O2 increased (3.96·10–5±1.99·10–6 mol/cm3), likewise, higher sulfates released (926.8±53.4 mg/L), increased mass loss (12.184±1.06%), rubber surface fragmentation, and important variations in the C–S, C=O bands, showing better results when devulcanization is carried out in synergism between HNO3 and H2O2.
Wenxin Gan, Hanyu Xue, Hongyi Lin, Renjin Gao, Yuchi Zhang, Liwei Wang, Jiuping Rao
Vol. 19., No.3., Pages 311-325, 2025
DOI: 10.3144/expresspolymlett.2025.22
Vol. 19., No.3., Pages 311-325, 2025
DOI: 10.3144/expresspolymlett.2025.22
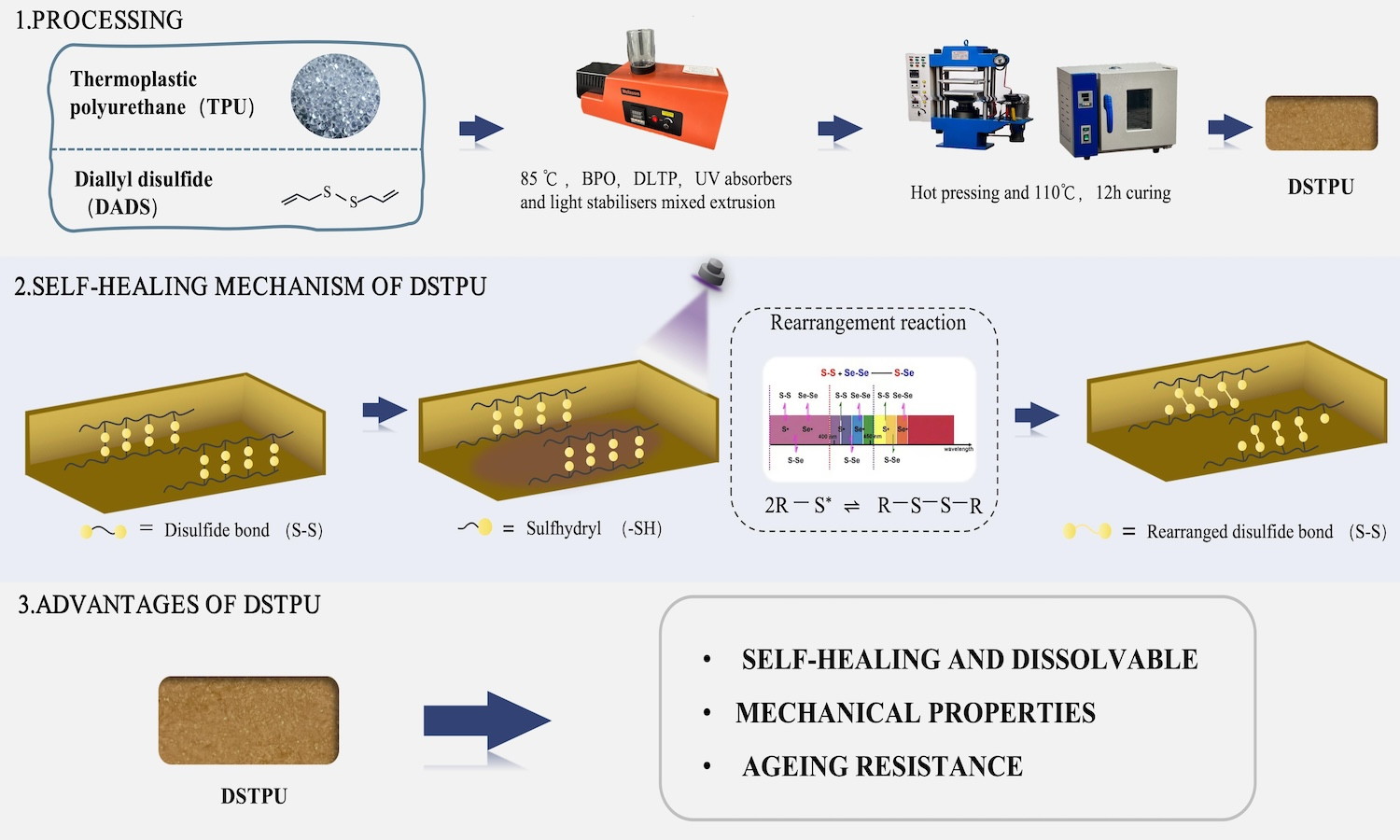
Cross-linking frequently enhanced the mechanical properties of linear polymeric materials; however, it also resulted in the transition from thermoplastic to thermosetting materials, which posed issues from an environmental perspective. Thermoplastic polyurethane (TPU) elastomers were extensively applied across various industries. To improve the mechanical properties of TPU while preserving its environmental benefits, this study integrated radical copolymerization technology to develop a reversible crosslinked TPU. Specifically, the linear polyurethane molecular chains were crosslinked using diallyl disulfide (DADS) as a functional cross-linking monomer. Through radical copolymerization reactions, reversible crosslinks formed from disulfide bonds were created between the linear polyurethane molecular chains, yielding a self-healing reversible crosslinked thermoplastic polyurethane (DSTPU). The study showed that DSTPU could self-heal and dissolve under UV light and alkaline N,N-dimethylformamide (DMF) conditions, achieving 82.2% self-healing efficiency at 3 phr DADS. It dissolved into fine particles in alkaline DMF. Disulfide bonds in DSTPU enhanced cross-linking, boosting 19% oxygen permeability, thermal conductivity (0.218 W/(m·K)), and mechanical properties like tensile stress (11.18 MPa), force (134.13 N), and elongation (548%). These bonds also enhanced aging resistance, cutting ΔYI to 6.0%.
Marek Pöschl, Radek Stoček, Petr Zádrapa, Martin Stěnička, Gert Heinrich
Vol. 18., No.12., Pages 1178-1190, 2024
DOI: 10.3144/expresspolymlett.2024.90
Vol. 18., No.12., Pages 1178-1190, 2024
DOI: 10.3144/expresspolymlett.2024.90
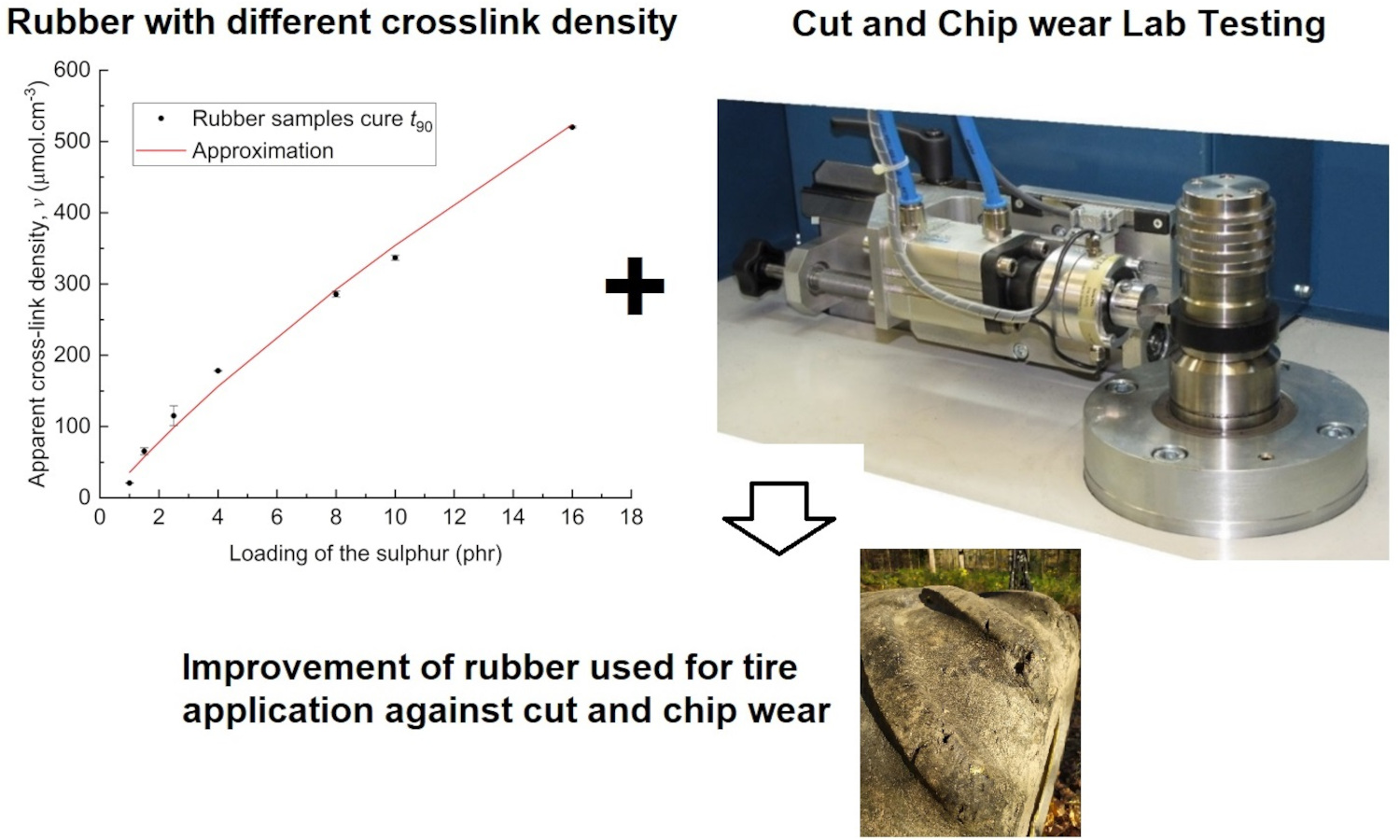
This paper extends previous studies by the authors that aimed to describe the effect of apparent cross-link density (CLD) of the rubber polymer networks on the fracture mechanism caused by cut and chip (CC) wear of natural rubber (NR), demonstrating the positive effect of conventional vulcanization (CV). This work is focused on the determination of the effect of CLD while keeping constant the accelerator-to-sulfur ratio A/S = 0.2, typical for CV systems. For this ratio, different sulfur quantities were chosen, and the concentration of the accelerator N-tert-butyl-benzothiazole sulphonamide (TBBS) was calculated to achieve CLDs in a range from 35 to 524 μmol・cm–3. Standard analyses such as tensile tests, hardness, rebound resilience and DIN abrasion were performed. From these analyses, the optimum physical properties of the NR-based rubber were estimated to be in the CLD range of approximately 60 to 160 μmol・cm–3. A CC wear analysis was performed with an Instrumented cut and chip analyzer (ICCA) and it was found that the highest CC wear resistance of the NR is in the CLD range of 35 to 100 μmol・cm–3. Furthermore, the effect of straininduced crystallization (SIC) of NR on CC wear and its dependence on the CLD region was discussed. For the first time, we determine a CLD range for a CV system in which the material achieves both optimal mechanical properties and CC wear resistance.
Sreethu Thiyyanthiruthy Kumbalaparambil, Ajay Haridas Chandaparambil, Kinsuk Naskar
Vol. 18., No.10., Pages 991-1007, 2024
DOI: 10.3144/expresspolymlett.2024.76
Vol. 18., No.10., Pages 991-1007, 2024
DOI: 10.3144/expresspolymlett.2024.76
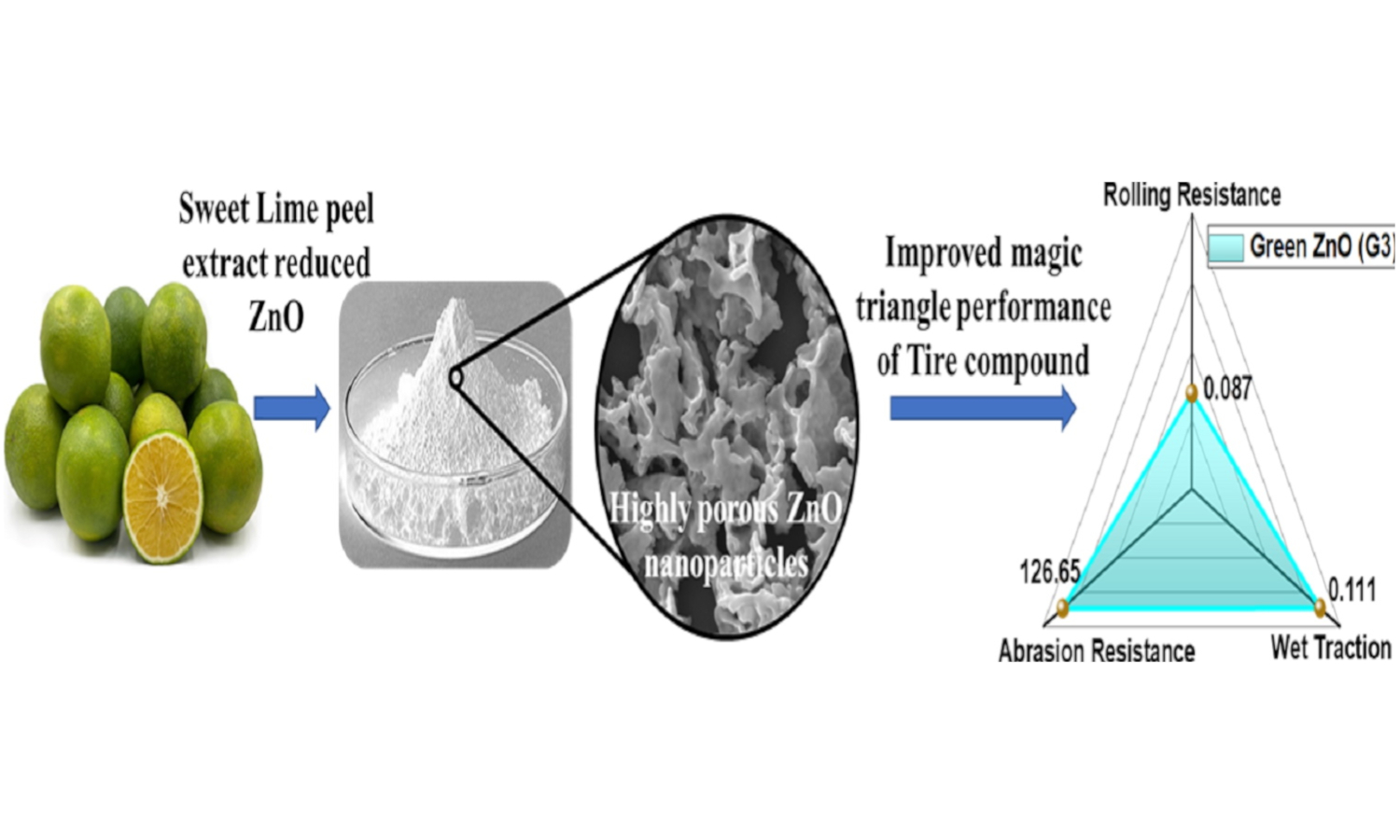
This study addresses the increasing demand for eco-friendly rubber compounding additives by exploring greensynthesized zinc oxide (ZnO) nanoparticles. The green synthesis of ZnO nanoparticles is gaining attention due to its ecofriendly approach and potential applications. This study investigates the synthesis of ZnO nanoparticles using sweet lime peel extract as a green method, comparing it with chemical synthesis. The obtained nanoparticles are characterized and evaluated for suitability as activators in natural rubber composites for tire applications. Furthermore, the cytotoxicity of the prepared ZnO nanoparticles on mice cells is assessed, revealing lower toxicity for green-synthesized ZnO compared to chemically synthesized ZnO. Payne effect analysis on the composites demonstrates improved polymer-filler interaction and mechanical properties for the green-synthesized ZnO-loaded composites. Notably, the incorporation of green-synthesized ZnO leads to significant enhancements in tensile strength due to its higher surface area. It achieves desirable magic triangle tire properties, including low rolling resistance, high wet traction, and high abrasion resistance. These findings highlight the promising potential of green ZnO as an environmentally friendly alternative to chemical ZnO in rubber compounding.


